
SINDROME X PLURIMETABÓLICO EN LA MUJER MENOPÁUSICA
|
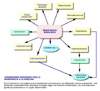
El síndrome X metabólico es una constelación de desórdenes metabólicos que resultan todos ellos de una resistencia a la insulina. Todas las anornalidades metabólicas asociadas al síndrome X conducen a desórdenes cardiovasculares. El riesgo de una enfermedad cardíaca y de una muerte prematura es muy elevado. Este síndrome se conoce también como síndrome metabólico, síndrome de resistencia a la insulina, síndrome dismetabólico, síndrome de obesidad o síndrome de Reaven. La definición del síndrome metabólica, producida en 2001 por un panel de expertos del Programa Nacional de Educación sobre el colesterol, NPEC (*) (EE.UU), es más estricto que las publicadas anteriormente por la OMS, el EGIR (European Group for the Study of Insulin Resistance) o el NHNES (National Health and Nutrition Examination Survey) (*). La Asociación Americana de Endocrinólogos Clínicos (AAEC), en agosto de 2002, extendió el concepto de resistencia a la insulina a otros componentes, como el síndrome del ovario poliquístico, el hígado graso de origen no alcohólico o la acantosis nigricans (*) El término síndrome X fué utilizado por primera vez por Reaven en 1988 para describir el riesgo cardiovascular que presentan algunos individuos con resistencia a la insulina. Estos pacientes se caracterizan además por hiperinsulinemia, son obesos, hipertensos y presentan dislipidemias, factores todos ellos derivados de la resistencia insulínica. La presencia de tres de los criterios señalados en la tabla 1 es suficiente para el diagnóstico de ests síndrome. Aunque la prevalencia del síndrome metabólico puede variar según la definición adoptada, se estima que varía entre el 7 y 36% en el hombre según la edad y entre el 5 y 22% para las mujeres entre los 40 y 55 años Las principales consecuencias clínicas del síndrome metabólico es la enfermedad arteriosclerótica cardiovascular y la mayor parte de los factores de riesgo del síndrome metabólico son igualmente factores de riesgo para la arteriosclerosis. Aunque la definición del síndrome metabólico no incluye otros factores de riesgo cardiovascular, algunos autores añaden algunos factores muy sensibles que pueden ayudar la definir el riesgo del paciente (*) |
|
La resistencia a la insulina se define como la disminución de la capacidad de la insulina para producir la respuesta fisiológica sobre el mantenimiento de la homeostasis de la glucosa en los tejidos diana (músculo esquelético, hígado, tejido adiposo). Como consecuencia, hay un incremento de la secreción de insulina con el fin de compensar la anterior situación, dando lugar a un hiperinsulinismo, que puede ser compatible con una glucemia plasmática normal. Cuando la cantidad de insulina segregada no es suficiente se desarrolla la diabetes tipo II. Adicionalmente, esta resistencia a la insulina es un factor desencadenante de otras alteraciones metabólicas (*) que suponen un riesgo cardiovascular como son la dislipidemia (aumento de los triglicéridos y de las LDLs, diminución de las HDLs) y la hipertensión. Existe un batería de pruebas para determinar la sensibilidad a la insulina, siendo el método de elección el de la perfusión euglucémica. Sin embargo, es un método costoso y lento y no siempre es aplicable a la población en general. El método HOMA (homeostasis model assessment) parece tener una fiabilidad bastante alta y se basa en la determinación de la glucosa e insulina en ayunas aplicando la fórmula: El CIGMA (continous infusion of glucose with model assessment) es otro modelo matemático alternativo al HOMA, donde se evalúan las concentraciones de glucosa e insulina en ayunas. En este método se administra una infusión continua de glucosa que se mantiene durante 1 h, tomándose tres muestras de glucosa e insulina en los últimos 10 min. Al término de la prueba se realiza también una prueba de glucosuria. Es un método sencillo y económico, pero, como el modelo anterior, es también de difícil comparación e interpretación. Otros métodos utilizados son el Modelo mínimo aproximado del metabolismo de
la glucosa, el Test de supresión de la insulina,y el Test de tolerancia a la insulina modificado. RESISTENCIA INSULÍNICA Y TEJIDO ADIPOSO El trastorno inicial de resistencia a la insulina parece situarse en el adipocito y consiste en una incapacidad para continuar almacenando ácidos grasos, secundaria a una predisposición genética, alteraciones dietéticas, etc.
|
REFERENCIAS
|
|
|
Obesity is a major risk factor for type 2 diabetes and cardiovascular disease. It also is an important component of metabolic syndrome, although in a minority of obese persons, insulin resistance does not develop. Insulin resistance may also develop in persons classified as lean by body mass index (BMI) standards, who could thus be considered "metabolically obese." Visceral adipose tissue has been proposed as the major site of fat deposition associated with the metabolic consequences of obesity.( n7 ) It is thought that visceral, or central, adiposity is the initial physical event that results in insulin resistance, by an increase in free fatty acid flux in portal and systemic circulations. Visceral adipose tissue may also contribute to other causes of increased atherosclerotic risk, including inflammatory, prothrombotic, and fibrinolytic factors (table 2). Whether measurement of these markers of adipose tissue hormonal activity improves accuracy in assessment of cardiovascular risk remains to be seen. Initial treatment of obesity associated with metabolic syndrome is lifestyle modification, followed by pharmacologic therapy. Pharmacologic agents available to treat obesity are of two classes, an appetite suppressant (sibutramine hydrochloride [Meridia]) and a drug that inhibits fat absorption (xenical [Orlistat]). Weight loss with these agents is modest, typically 5% to 10% of initial weight. Of considerable interest are newer agents that target endogenous cannabinoid receptors to inhibit both short- and long-term food intake. Rimonabant is currently in phase 3 clinical trials. Surgical therapies such as gastric banding and gastric bypass can be considered for patients who are severely obese (BMI =40 kg/m²) or for patients with a BMI of 35 kg/m² or more who have comorbid conditions. This latter group includes the majority of persons with metabolic syndrome . DyslipidemiaCombined hyperlipidemia is a hallmark of metabolic syndrome . The characteristic lipid disorders seen in this syndrome are hypertriglyceridemia, low levels of high-density lipoprotein cholesterol (HDL-C) and, often, normal levels of low-density lipoprotein cholesterol (LDL-C), although the LDL-C particles are typically smaller and more dense than usual. Whether these small, dense LDL-C particles confer increased cardiovascular risk independent of that related to hypertriglyceridemia and low HDL-C levels is uncertain.( n8 ) The diagnosis of dyslipidemia is best made when a patient is in a basal state without acute illness. Low HDL-C levels (<40 mg/dL [1.03 mmol/L] in men, <50 mg/dL [1.29 mmol/L] in women) are altered little by fasting. However, triglyceride concentration can rise substantially after food intake, and current diagnostic criteria are based on measurements obtained after 12 hours of fasting. It is important that physicians be mindful of other conditions that result in low HDL-C levels and to screen for these disorders when appropriate. Hypothyroidism, glucocorticoid excess (endogenous and exogenous), and use of protease inhibitors can result in lipid abnormalities typical of those seen in metabolic syndrome . Treatment of the dyslipidemia of metabolic syndrome should involve nonpharmacologic interventions, including weight loss, exercise, and a low-fat diet. Reducing LDL-C levels with use of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors ("statins") is also appropriate for patients with metabolic syndrome . The ATP III guidelines recommend that LDL-C be the primary target of lipid-lowering therapy when a patient's triglyceride level is below 500 mg/dL (5.65 mmol/L). Metabolic syndrome can be considered a coronary artery disease (CAD) equivalent. Thus, it is appropriate to have target LDL-C levels that are below 100 mg/dL (2.59 mmol/L). Achieving this goal usually requires addition of a cholesterol-lowering agent, such as a statin. However, for many patients, statin therapy does not correct abnormalities of triglyceride and HDL-C concentrations. Modifying triglyceride and HDL-C levels with drug therapy improves cardiovascular risk beyond the benefits achieved with statins alone. The Coronary Drug Project( n9 ) and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VAHIT)( n10 ) used drug interventions (niacin and gemfibrozil) designed to modify triglyceride and HDL-C levels, and these interventions were associated with a reduction in cardiovascular events in both studies. Caution should be exercised when using both a fibric acid derivative and a statin, because the risk of myositis is increased with combination therapy. In addition, creatine kinase levels should be monitored if symptoms such as myalgia develop, especially in the setting of combination therapy. HypertensionHypertension is an important hallmark of metabolic syndrome . Weight gain in youth( n11 ) and middle age( n12 ) is positively correlated with blood pressure levels, and weight reduction can effectively lower blood pressure in overweight hypertensive patients.( n13 ) This scenario again demonstrates the overlap of individual components of metabolic syndrome . The ATP III defines elevated blood pressure as a reading of 130/85 mm Hg or greater. This category includes patients taking antihypertensive medicines even if treatment achieves a blood pressure level that is within target range. As with: dyslipidemia, the initial treatment of hypertension is nonpharmacologic therapy, including sodium restriction and weight loss through calorie restriction and exercise. However, pharmacologic therapy is required in many patients with hypertension. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure( n14 ) provides guidelines for intensive treatment of hypertension. Although use of thiazide diuretics and ß-blockers has been avoided in patients with glucose tolerance abnormalities, the safety and efficacy of such medications have been demonstrated in large clinical trials.( n15 ) Drugs of these classes can be used in treatment of hypertension in patients with metabolic syndrome . The mechanism supporting the association between insulin resistance and hypertension is unclear. Insulin acts as a direct vasodilator when given as a short-term infusion( n16 ) but also acts to increase both sympathetic outflow( n17 ) and renal sodium reabsorption.( n18 ) These last two mechanisms may counter vasodilatory effects and result- in elevations of blood pressure in the insulin-resistant state. Despite evidence that blood pressure control is critical for reducing cardiovascular risk in diabetic patients, clinical studies show that fewer diabetic patients than nondiabetic patients in a matched group have blood pressures under 140/90 mm Hg. Abnormal glucose toleranceElevated fasting glucose levels are an important feature of metabolic syndrome , but neither impaired fasting glucose nor diabetes is an absolute criterion. Insulin resistance is associated with an increased risk of type 2 diabetes, and overt diabetes develops in many persons with metabolic syndrome . Measurement of fasting glucose is required in all patients with metabolic syndrome to identify comorbid conditions that warrant specific intervention. Currently, impaired fasting glucose is identified by a fasting blood glucose level of 100 to 126 mg/dL (5.6 to 7.0 mmol/L), a criterion recently reduced from a level of 110 to 126 mg/dL (6.1 to 7.0 mmol/L). While NCEP guidelines suggest measurement of fasting glucose when a diagnosis of metabolic syndrome is being considered, the role of fasting insulin levels, postchallenge glucose levels, and more precise measures of insulin sensitivity is open to question. At this time, these measures are not part of the diagnostic criteria for metabolic syndrome and are often more useful for epidemiologic or research considerations (see article by Dr Sivitz on page 41). Again, nonpharmacologic intervention is appropriate as initial therapy for patients with impaired fasting glucose or impaired glucose tolerance, but insulin-sensitizing drugs may be considered an early treatment option if lifestyle intervention is ineffective, even in the absence of overt diabetes. The Diabetes Prevention Program demonstrated the effectiveness of lifestyle change (weight loss of 7% and exercise level of 150 min/wk) in persons with impaired fasting glucose, but metformin hydrochloride (850 mg twice daily) was also effective in delaying progression to overt diabetes in patients with impaired fasting glucose.( n19 ) Polycystic ovary syndromePolycystic ovary syndrome affects 5% to 10% of US women of reproductive age and has been increasing in incidence similarly to metabolic syndrome and type 2 diabetes. It is characterized by anovulation with irregular menses, infertility, and androgen excess leading to hirsutism and acne. Polycystic ovary syndrome , like metabolic syndrome , is associated with insulin resistance and an increased risk of type 2 diabetes and cardiovascular disease. By age 40, 40% of women with polycystic ovary syndrome have evidence of abnormal glucose tolerance.( n20 ) Use of insulin-sensitizing drugs to treat the anovulation and infertility of polycystic ovary syndrome is of considerable interest. A meta-analysis of metformin use in polycystic ovary syndrome ( n21 ) demonstrated that 46% of subjects who received metformin monotherapy achieved ovulation, compared with 24% of those who received placebo. Therapy with metformin (Glucophage) also enhances the effectiveness of clomiphene citrate (Clomid, Milophene, Serophene) in inducing ovulation and fertility. However, similar to the Diabetes Prevention Program findings, equal or better ovulation rates with regular menses and decreased hair growth are achieved through lifestyle modification than through metformin monotherapy. Atherosclerotic cardiovascular diseaseThere is no question that impaired fasting glucose and impaired glucose tolerance represent intermediate stages in the progression from metabolic syndrome to type 2 diabetes. The process is considered a continuum in which progressive defects in insulin action and insulin release lead to more overt abnormalities of glucose homeostasis.( n22 ) Impaired glucose tolerance has also been associated with CAD and stroke before it progresses to full-blown diabetes. In the Funagata Diabetes Study,( n23 ) mortality rates for cardiovascular disease were significantly higher in subjects with impaired glucose tolerance than in those with normal glucose tolerance, although lesser degrees of glucose intolerance (impaired fasting glucose) were not associated with increased risk. In the Risk Factors in Impaired Glucose Tolerance for Atherosclerosis and Diabetes (RIAD) trial,( n24 ) 2-hour postchallenge glucose concentrations correlated more closely with carotid intima-media thickness than did fasting glucose concentrations in patients with a family history of type 2 diabetes. Impaired fasting glucose and type 2 diabetes have insulin resistance in common, and insulin resistance has been shown to be an independent risk factor for atherosclerotic cardiovascular disease.( n25 ) It is not unexpected that in the natural history of type 2 diabetes, macrovascular complications may antedate--often by many years--the typical diagnosis of diabetes. Thus, the challenge is to identify and correct defects in glucose metabolism as early as possible. TherapyCurrent recommendations for therapy for metabolic syndrome focus on correction of the components (ie, hypertension, dyslipidemia, visceral adiposity, and abnormal glucose tolerance). It has been said many times and in many ways, but the fact remains that exercise, diet, and weight loss each independently improves insulin resistance and reduces progression to type 2 diabetes. Even though the success of lifestyle modification is limited, the importance of such therapy cannot be overemphasized. Drug intervention focuses on treatment of the manifestations of metabolic syndrome with clearly defined targets for blood pressure, weight, and levels of triglycerides, HDL-C, and hemoglobin A[sub1c]. The success of metformin use in polycystic ovary syndrome and in the Diabetes Prevention Program suggests that one medication or drug class could be used to treat the entire syndrome . Use of medications in the thiazolidinedione class (pioglitazone hydrochloride [Actos] and rosiglitazone maleate [Avandia]) directly improves insulin resistance but has not yet been shown to reduce cardiovascular morbidity or mortality. Thus, the approach to treatment of metabolic syndrome is aggressive management of its individual components. ConclusionMetabolic syndrome represents a clustering of cardiovascular risk factors linked through their association with insulin resistance. Since insulin resistance is an independent risk factor for cardiovascular disease, its presence can lead to macrovascular complications long before other features of metabolic syndrome are evident. Challenges remaining in the identification of high-risk persons include the introduction of clinical markers of insulin resistance, integration of postchallenge glucose and lipid concentrations, and better definition of the role of inflammatory, prothrombotic, and genetic factors. Improved understanding of the risk factors for metabolic syndrome is required, and clinical trials of therapeutic interventions specifically targeted to this syndrome need to be conducted. Earn CME credit on the Web. www.postgradmed.com The author discloses no financial interests in this article and no unlabeled uses of any product mentioned. |





